ok this is understandable, you went from crappy quality of life, RX and injectibles with so many prescriptions and you only take 4-6 of calcium that is very different than 12, you can see the concerrn with just one. That was my concern the total number of just one mineral, not that 12 was a lot. You sound like you are doing great and like you I would also pick your life post op too.@Sue I was on 19 prescriptions meds a day before my DS, including at least 4 daily insulin injections. I'll swallow as many supplement pills as I have to in order to maintain health. It's a lot better than being dead.
That being said, I take 4-6 calcium pills per day. Country Life Target Mins Cal-Mag Complex, which is a combination of three different kinds of calcium, 1,000 mg per 2 pill dose. I have never bought into that no more than 600 mg calcium per dose thing, and it seems to be working for me.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
PatchMD who has used them, reviews?
- Thread starter Sue
- Start date

Help Support Bariatric & Weight Loss Surgery Forum:
I am very familiar with vitalady DS schedule list.This seriously concerns me. You need to read the forum Vitamins & Labs. I take 20 pills before noon everyday. If you are this nervous, I don't think the DS is for you.
my concern was 12 OF ONE PILL, I had not read anyone that takes that many, which I thought was a lot, my post was questioning 12 of just calcium, not the total amount, and it is a viable concern
No that makes sense!!! Holy crap, whowowwh that is a lot of pills, just for calcium, but I understand you need them for your calcium issue. Yes I am familiar with vitalady I had counted about 28 or so, so not far from what you posted, I was asuming around there, which is very doable. I know I will have to move things around depending on my specific results. thanks for the clarification on why you take so many calciums.no 12 calcium pills per day is not typical, metabolic bone disease, so I need more calcium (and magnesium because of calcium) , vitD3, so my count is higher than most, you will be fine. Vitalady plan is only about 30 total/day, totally doable.
Well, it all depends on YOUR labs.I am very familiar with vitalady DS schedule list.
my concern was 12 OF ONE PILL, I had not read anyone that takes that many, which I thought was a lot, my post was questioning 12 of just calcium, not the total amount, and it is a viable concern
Most of us need to start with the Vitalady list and then when post-op labs start rolling in, you adjust.
As for the 12 of calcium...it depends on how much calcium citrate is in ONE pill. Most we typically take at one time is 600 mgs since more than that is supposedly not used. But each one is different. Some take three at one time, most take 2.
I'm an oddball in that *I* only take 2 calcium a day and my labs for calcium are mid range normal. BUT vitamins are critical. You can DIE from not paying attention to your vitamins and your own labs.
@southernlady yes I understand, it is individualized, but still not the average to take 12 calciums/day and I understand why @revisionDS needs to take that many due to bone issues. Any you just validated my question, such a difference, I just wanted to make sure 12 pills was NOT typical just for calcium, not 12 was total, I get that!!!!
revisionDS
Well-Known Member
- Joined
- Sep 25, 2014
- Messages
- 208
I take 12 citrical Max throughout the day. That is 3780 mg of elemental calcium citrate per day. You can get more information on them at: http://labeling.bayercare.com/omr/online/citracal-maximum.pdf I would rather take @southernlady 2 calcium/day, but obviosuly I can't do that. I think both southernlady and myself are not typical, we are extremes, and I would say the typical is more like vitalady with the 4-6/day calcium. I understand your concern there is a 10 pill difference just in this one pill, calcium between southernlady and myself, and that was your original concern. Yes you need to go by your own lab work results and adjust, you won't know what that is, and it will change over time, etc. I knew this going into the DS, anyone with metabolic bone disease going into a malabsorption surgery needs to think long and hard about this, DS didn't cause this but it sure does make it more difficult to treat. Don't worry about the number of pills, we all have concerns over this, but it is a small price to pay for a good quality of life as @Elizabeth N. said in her post.

$44.75 ($1.23 / Ounce)
$54.75 ($1.50 / Ounce)
LIVING HEALTHY NUTRITION BariSuccess Vanilla Whey Isolate Protein Powder - 30g Protein, 30 Servings/Container - Free of Fat, Sugar, Gluten, Soy & Lactose - Low Carb Bariatric Meal Replacement Protein
My Bariatric Kitchen

$19.87 ($0.17 / Count)
$29.97 ($0.25 / Count)
PAWFECTCHEW Pawfect Mobility - Glucosamine Treats for Dogs - Hip & Joint Health Supplement Chews w/Omega-3, Chondroitin, MSM - Made in USA - Joint Pain Relief - Hip & Joint Care - 120ct
PawfectSupplies

$10.00 ($10.00 / Count)
First Days Maternity - Maxi Peri Bottle for Maximum Postpartum Soothing! Large 650ml Capacity Allows for an Effective Soothe. Pink Colour
First Days Maternity

$41.00
Walttools Tru Tex CORAL Texture Roller Sleeve for Concrete Flatwork and Vertical Concrete - Quick, Easy, Realistic Patterns, User-Friendly (Coral Stone)
Decorative Concrete Supply Source

$16.99 ($0.39 / Ounce)
BariWise Protein Soup Mix, Chicken Noodle, 15g Protein, Low Carb (7ct).
DIET DIRECT

$11.36
$14.95
Gastric Bypass Diet: Step By Step Guide to Gastric Bypass Surgery (Bariatric Cookbook)
Amazon.com

$9.49
$15.99
Fresh Start Bariatric Cookbook: Healthy Recipes to Enjoy Favorite Foods After Weight-Loss Surgery
Amazon.com

$10.17
$18.99
The Complete Bariatric Cookbook and Meal Plan: Recipes and Guidance for Life Before and After Surgery
Amazon.com

$18.70 ($1.65 / Ounce)
$19.99 ($1.76 / Ounce)
Quest Nutrition Mini Cookies & Cream Protein Bars, 8g Protein, 1g Sugar, 2g Net Carbs, Gluten Free, 14 Count
Amazon.com

$8.99
$15.99
The Gastric Sleeve Bariatric Cookbook: Easy Meal Plans and Recipes to Eat Well & Keep the Weight Off
Amazon.com

$9.29
$14.95
Gastric Bypass Recipes: 80+ Simple Recipes for the First Stage After Gastric Bypass Surgery (Bariatric Cookbook)
Amazon.com
Elizabeth N.
Herder of cats
Yeah, I take roughly the same number of pills per day, maybe a couple more, as I did before my surgery. The calcium I use is one hell of a horse pill, so some folks would want to cut them up or let them fall apart in a little water.ok this is understandable, you went from crappy quality of life, RX and injectibles with so many prescriptions and you only take 4-6 of calcium that is very different than 12, you can see the concerrn with just one. That was my concern the total number of just one mineral, not that 12 was a lot. You sound like you are doing great and like you I would also pick your life post op too.
newanatomy
Well-Known Member
- Joined
- Jan 5, 2014
- Messages
- 2,983
I went to the Patch MD site to order the calcium, it is on back order, shit. They better do a better job than that if they want my business.
Bagaof4
Well-Known Member
I sent emails to the owner to ask about the high levels of B6 in the multi patch and received this respond. Unhappy with what I consider to be a "non response" as I will NOT be relying on my doctor about any vitamin levels. Guess I will have to wait and see what 25 mg a day of B6 does to the labs of those brave enough to start the patches.
Am I being overly paranoid?
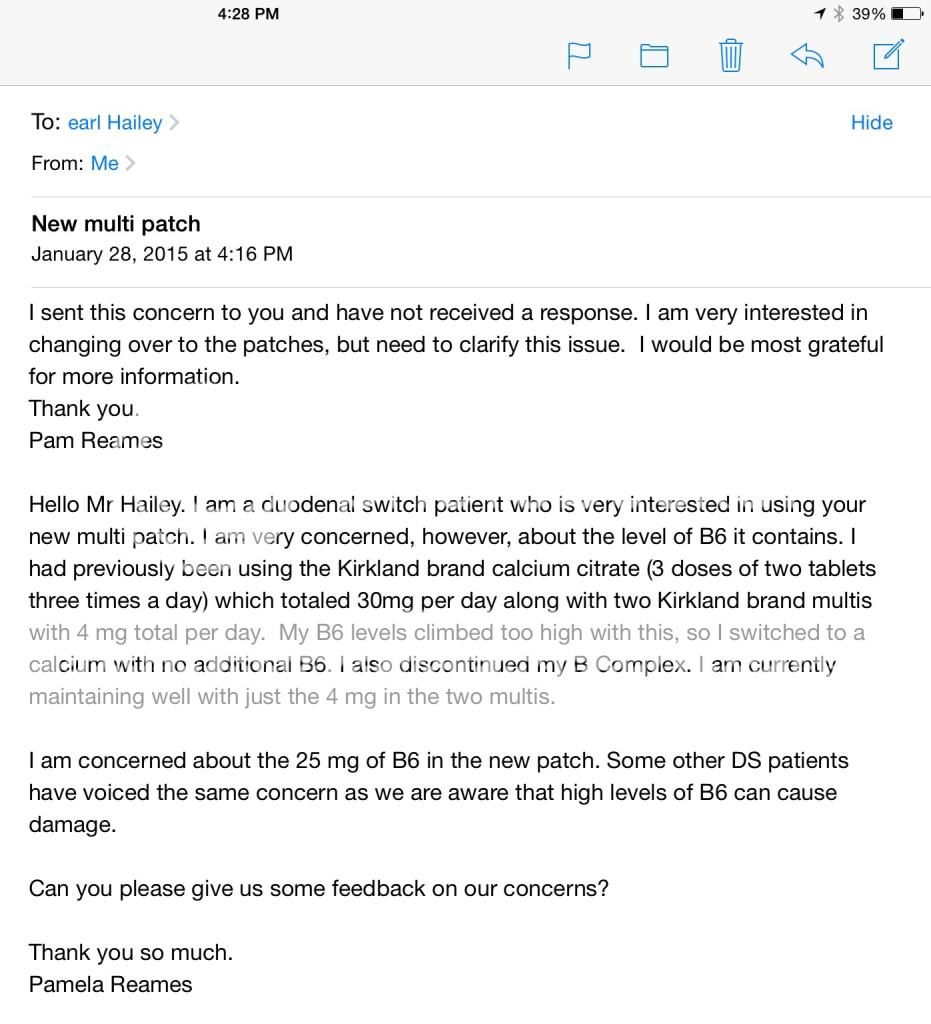
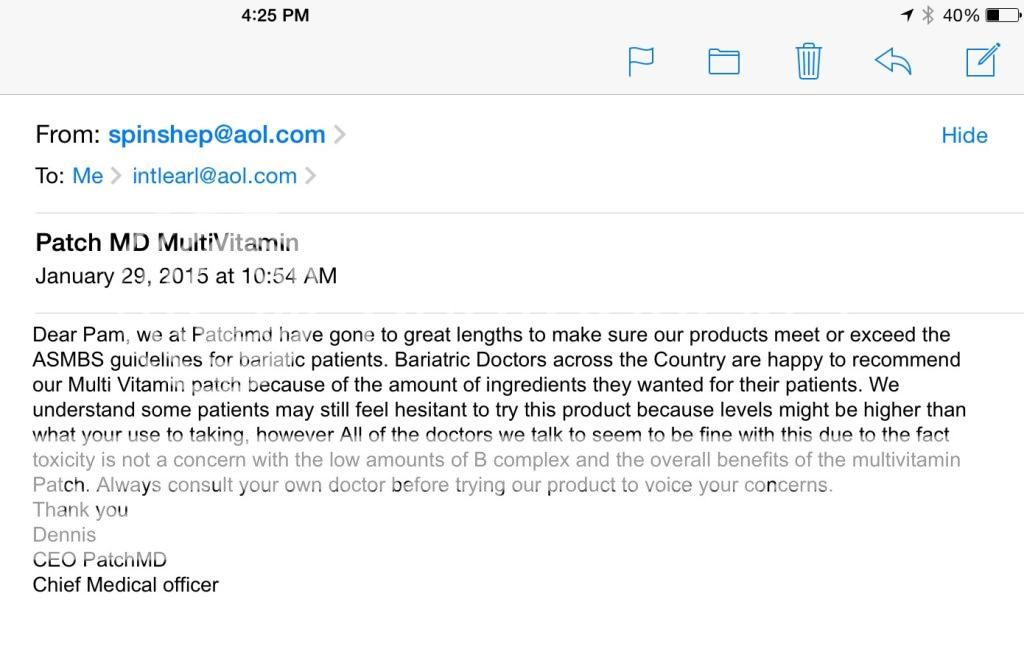
Am I being overly paranoid?


.. what "YOUR USE" to taking ... ?? Grammar fingernails on my spinal column!
That is a non-answer, as you said.
However, I have suspected for a long time that the Costco calciums have the WRONG dose of B6 in them, since pretty much ALL of us went too high on them. I think it is something particular to those tablets - and you could probably safely try the patches.
But I still don't see how all of these things get passed through the skin efficiently. Fat solubles, OK - but the rest? I want a scientific explanation of how the minerals and complex water soluble vitamins get across the dermis.
That is a non-answer, as you said.
However, I have suspected for a long time that the Costco calciums have the WRONG dose of B6 in them, since pretty much ALL of us went too high on them. I think it is something particular to those tablets - and you could probably safely try the patches.
But I still don't see how all of these things get passed through the skin efficiently. Fat solubles, OK - but the rest? I want a scientific explanation of how the minerals and complex water soluble vitamins get across the dermis.
Last edited:
newanatomy
Well-Known Member
- Joined
- Jan 5, 2014
- Messages
- 2,983
Bagaof4
Well-Known Member
Thanks Diana. Just another reason I will wait to see lab results of those who are switching over.
This is just a thought. Regarding the over the RDA amounts. Is it possible RDA is based on the assumption that people absorb certain amounts in their food and it is to supplement what we are supposed to get in our food. The larger amounts are really for those that malabsorb. Just a thought, I'm not good at this stuff but it's just a thought I had.
Saying that. With the ~1000 mgs from the multi and the 1000 mgs from the calcium/d3 patch, theoretically we would not need to take any calcium. If the iron is absorbed through the skin in the multi, we would have no need for iron supplementation. So with those two patches, what else would we need if we absorb what is in the patch. I'm looking at costs as well as whether or not it would work. I think we're going to have to wait for those who are trying it to get their labs back
Saying that. With the ~1000 mgs from the multi and the 1000 mgs from the calcium/d3 patch, theoretically we would not need to take any calcium. If the iron is absorbed through the skin in the multi, we would have no need for iron supplementation. So with those two patches, what else would we need if we absorb what is in the patch. I'm looking at costs as well as whether or not it would work. I think we're going to have to wait for those who are trying it to get their labs back
I just did a little research on PubMed, looking for reports of clinical studies on transdermal vitamin and mineral delivery systems. I continue to have doubts about the PatchMD system. But here are a few articles, and links to the searches so you can peruse the results yourself:
Vitamin + (transdermal delivery): http://www.ncbi.nlm.nih.gov/pubmed/?term=vitamin+"transdermal+delivery"
AAPS PharmSciTech. 2015 Jan 22. [Epub ahead of print]
Investigating Transdermal Delivery of Vitamin D3.
Alsaqr A1, Rasoully M, Musteata FM.
Author information
Transdermal delivery of therapeutic amounts of vitamin D3 is proposed to overcome its variable oral bioavailability, especially for people who suffer from fat malabsorption. The main challenge for this delivery route is to overcome the barrier properties of skin, especially for very lipophilic compounds such as vitamin D3. In this study, the effect of different penetration enhancers, such as oleic acid, dodecylamine, ethanol, oleic acid in propylene glycol, isopropyl myristate, octyldodecanol, and oleyl alcohol in propylene glycol were evaluated in vitro for their effectiveness in deliveringvitamin D3 through polyamide filter, polydimethylsiloxane membrane, and porcine skin. A diffusion cell was used to study the transdermal permeability of vitamin D3. Ointment formulations of vitamin D3 were prepared containing the most widely used penetration enhancers, oleic acid, and dodecylamine. The ointment containing oleic acid as chemical penetration enhancer did not improve delivery compared to control. On the other hand, the formulation containing dodecylamine as a penetration enhancer did improve the transdermal delivery of vitamin D3. However, statistical significance and an amount high enough for nutritional supplementation purposes were reached only when the skin was pretreated with 50% ethanol. In these conditions, the ointment delivered an amount of 760-ng vitamin D3 per cm2 of skin. The research shows promise that transdermal delivery could be an effective administration route for vitamin D3 when ethanol and dodecylamine are used as penetration enhancers.
This one has the full-length article available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3976443/
Int J Biomed Sci. 2014 Mar;10(1):21-4.
Topical delivery of vitamin d3: a randomized controlled pilot study.
Sadat-Ali M1, Bubshait DA1, Al-Turki HA1, Al-Dakheel DA1, Al-Olayani WS1.
Author information
BACKGROUND AND OBJECTIVE:
The purpose of the present study is to explore the assessment if the transdermal delivery of vitamin D is feasible.
METHODS:
In 50 female Medical students, this study was conducted. Age, weight and height was taken, a detailed history and clinical examination was performed. Blood was drawn for 25 Hydroxy Vitamin D3 (25OHD) level. Two women had >30 ng/mL of 25OHD and was excluded from the study. The participants were divided into two groups of 24 in each arm. All participants equivocally agreed not to change their dietary habits and life style till the study was over. The study group of women were asked to apply; Top-D (Aloe Vera based- Vitamin D3) (Patency Pending) was developed at King Fahd Hospital of the University, AlKhobar with each gram of the Top-D cream delivering 5000 IU of vitamin D3. The second group used 1 gram of Aloe vera gel. The participants had no knowledge to which group they belong. A second blood sample was taken at the end of 3 months and the data was analyzed.
RESULTS:
The data of 48 women was available for analysis. The average age was 22.58 ± 1.95 years. The mean pre-treatment 25OHD in the study group was 12.05 ng/Ml ± 6.54 and post-treatment was 37.95 ng/mL ± 6.43 (P=0.001, CI<28.582 ). In control group pre-treatment 25OHD was 11.4 ng/mL ± 3.97 and post-treatment was 10.58ng/mL ± 3.03.
CONCLUSIONS:
This randomized control study shows that vitamin D3 can safely be delivered through the dermal route. This route could be exploited in treating vitamin D deficiency.
Calcium + (transdermal delivery): http://www.ncbi.nlm.nih.gov/pubmed/?term=calcium+"transdermal+delivery" NO RELEVANT RESULTS
Iron + (transdermal delivery): http://www.ncbi.nlm.nih.gov/pubmed/?term=iron+"transdermal+delivery"
Full length article - in rats - required prepping the skin with needles: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578117/
Pharm Res. 2013 Mar;30(3):889-98. doi: 10.1007/s11095-012-0930-2. Epub 2012 Nov 28.
Microporation and 'iron'tophoresis for treating iron deficiency anemia.
Modepalli N1, Jo S, Repka MA, Murthy SN.
Author information
Abstract
PURPOSE:
Iontophoretic mediated transdermal delivery of ferric pyrophosphate (FPP) in combination with microneedle pretreatment was investigated as a potential treatment for iron deficiency anemia (IDA).
METHODS:
In vitro transdermal delivery studies were performed using hairless rat skin and pharmacodynamic studies were performed in hairless anemic rat model. The hematological and biochemical parameters like hemoglobin, hematocrit and % serum transferrin were monitored in rats at healthy, anemic condition and post treatment. Micropores created by the microneedles were visualized in histological skin sections after staining with hemotoxylin and eosin. The recovery of micropores was investigated in vivo by measuring Transepidermal water loss (TEWL) at different time points.
RESULTS:
The passive, microneedle and iontophoresis mediated delivery did not lead to significant improvement in hematological and biochemical parameters in anemic rats, when used individually. When iontophoresis (0.15 mA/cm(2) for 4 hours) was combined with microneedle pretreatment (for 2 min), therapeutically adequate amount of FPP was delivered and there was significant recovery of rats from IDA.
CONCLUSIONS:
Microneedle and iontophoresis mediated delivery of iron via transdermal route could be developed as a potential treatment for IDA. The transdermal controlled delivery of iron could become a potential, safe and effective alternative to parenteral iron therapy.
I did find this interesting 2014 article about vitamin D supplementation - I don't know why it was published in the Journal of Animal Science, however! https://www.animalsciencepublications.org/publications/jas/articles/92/3/893
Vitamin + (transdermal delivery): http://www.ncbi.nlm.nih.gov/pubmed/?term=vitamin+"transdermal+delivery"
AAPS PharmSciTech. 2015 Jan 22. [Epub ahead of print]
Investigating Transdermal Delivery of Vitamin D3.
Alsaqr A1, Rasoully M, Musteata FM.
Author information
- 1Department of Pharmaceutical Sciences, Albany College of Pharmacy and Health Sciences, 106 New Scotland Avenue, Albany, New York, 12208, USA.
Transdermal delivery of therapeutic amounts of vitamin D3 is proposed to overcome its variable oral bioavailability, especially for people who suffer from fat malabsorption. The main challenge for this delivery route is to overcome the barrier properties of skin, especially for very lipophilic compounds such as vitamin D3. In this study, the effect of different penetration enhancers, such as oleic acid, dodecylamine, ethanol, oleic acid in propylene glycol, isopropyl myristate, octyldodecanol, and oleyl alcohol in propylene glycol were evaluated in vitro for their effectiveness in deliveringvitamin D3 through polyamide filter, polydimethylsiloxane membrane, and porcine skin. A diffusion cell was used to study the transdermal permeability of vitamin D3. Ointment formulations of vitamin D3 were prepared containing the most widely used penetration enhancers, oleic acid, and dodecylamine. The ointment containing oleic acid as chemical penetration enhancer did not improve delivery compared to control. On the other hand, the formulation containing dodecylamine as a penetration enhancer did improve the transdermal delivery of vitamin D3. However, statistical significance and an amount high enough for nutritional supplementation purposes were reached only when the skin was pretreated with 50% ethanol. In these conditions, the ointment delivered an amount of 760-ng vitamin D3 per cm2 of skin. The research shows promise that transdermal delivery could be an effective administration route for vitamin D3 when ethanol and dodecylamine are used as penetration enhancers.
This one has the full-length article available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3976443/
Int J Biomed Sci. 2014 Mar;10(1):21-4.
Topical delivery of vitamin d3: a randomized controlled pilot study.
Sadat-Ali M1, Bubshait DA1, Al-Turki HA1, Al-Dakheel DA1, Al-Olayani WS1.
Author information
- 1College of Medicine, University of Dammam, King Fahd Hospital of the University, AlKhobar, Saudi Arabia.
BACKGROUND AND OBJECTIVE:
The purpose of the present study is to explore the assessment if the transdermal delivery of vitamin D is feasible.
METHODS:
In 50 female Medical students, this study was conducted. Age, weight and height was taken, a detailed history and clinical examination was performed. Blood was drawn for 25 Hydroxy Vitamin D3 (25OHD) level. Two women had >30 ng/mL of 25OHD and was excluded from the study. The participants were divided into two groups of 24 in each arm. All participants equivocally agreed not to change their dietary habits and life style till the study was over. The study group of women were asked to apply; Top-D (Aloe Vera based- Vitamin D3) (Patency Pending) was developed at King Fahd Hospital of the University, AlKhobar with each gram of the Top-D cream delivering 5000 IU of vitamin D3. The second group used 1 gram of Aloe vera gel. The participants had no knowledge to which group they belong. A second blood sample was taken at the end of 3 months and the data was analyzed.
RESULTS:
The data of 48 women was available for analysis. The average age was 22.58 ± 1.95 years. The mean pre-treatment 25OHD in the study group was 12.05 ng/Ml ± 6.54 and post-treatment was 37.95 ng/mL ± 6.43 (P=0.001, CI<28.582 ). In control group pre-treatment 25OHD was 11.4 ng/mL ± 3.97 and post-treatment was 10.58ng/mL ± 3.03.
CONCLUSIONS:
This randomized control study shows that vitamin D3 can safely be delivered through the dermal route. This route could be exploited in treating vitamin D deficiency.
Calcium + (transdermal delivery): http://www.ncbi.nlm.nih.gov/pubmed/?term=calcium+"transdermal+delivery" NO RELEVANT RESULTS
Iron + (transdermal delivery): http://www.ncbi.nlm.nih.gov/pubmed/?term=iron+"transdermal+delivery"
Full length article - in rats - required prepping the skin with needles: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578117/
Pharm Res. 2013 Mar;30(3):889-98. doi: 10.1007/s11095-012-0930-2. Epub 2012 Nov 28.
Microporation and 'iron'tophoresis for treating iron deficiency anemia.
Modepalli N1, Jo S, Repka MA, Murthy SN.
Author information
Abstract
PURPOSE:
Iontophoretic mediated transdermal delivery of ferric pyrophosphate (FPP) in combination with microneedle pretreatment was investigated as a potential treatment for iron deficiency anemia (IDA).
METHODS:
In vitro transdermal delivery studies were performed using hairless rat skin and pharmacodynamic studies were performed in hairless anemic rat model. The hematological and biochemical parameters like hemoglobin, hematocrit and % serum transferrin were monitored in rats at healthy, anemic condition and post treatment. Micropores created by the microneedles were visualized in histological skin sections after staining with hemotoxylin and eosin. The recovery of micropores was investigated in vivo by measuring Transepidermal water loss (TEWL) at different time points.
RESULTS:
The passive, microneedle and iontophoresis mediated delivery did not lead to significant improvement in hematological and biochemical parameters in anemic rats, when used individually. When iontophoresis (0.15 mA/cm(2) for 4 hours) was combined with microneedle pretreatment (for 2 min), therapeutically adequate amount of FPP was delivered and there was significant recovery of rats from IDA.
CONCLUSIONS:
Microneedle and iontophoresis mediated delivery of iron via transdermal route could be developed as a potential treatment for IDA. The transdermal controlled delivery of iron could become a potential, safe and effective alternative to parenteral iron therapy.
I did find this interesting 2014 article about vitamin D supplementation - I don't know why it was published in the Journal of Animal Science, however! https://www.animalsciencepublications.org/publications/jas/articles/92/3/893
Similar threads
- Replies
- 6
- Views
- 390
- Replies
- 8
- Views
- 1K































