huneypie
Wannabe
I added some protein powder to some low carb real egg custard and wondered if it's OK to count it.

Why would you think it DOESN'T count?
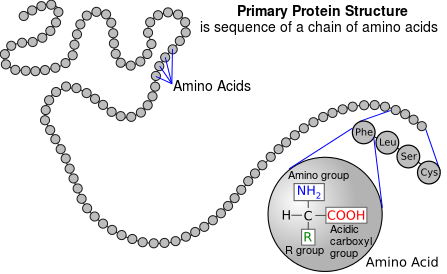
Proteins are strings of amino acids. To digest them, the bonds between the amino acids have to be broken (hydrolyzed) to release the amino acids, which are then absorbed out of the intestine. The amino acids in the protein in your food are then reassembled into YOUR proteins.
When you cook proteins, they denature - which means that they are irreversibly transformed from the carefully folded versions which are biologically active (as enzymes or structural proteins) into "dead" versions. The amino acids still are bonded together, and need to be digested (hydrolyzed to amino acids) to be used - so while they are cooked/denatured, and thus "dead" in terms of their biological function, as FOOD, they are just the same as before being cooked in their value to you. Think of the "live" protein as a Rubik's cube when it is in the goal position (all the same color on each side), and a denatured protein as a cube after a bunch of random rotations - all the same pieces are there, but the protein won't "work" - it's dead. But the pieces are all still there - and since it is as separate pieces (the amino acids) that they are of any use to you, it does not matter whether the protein is raw (alive) or cooked (dead) - the value to YOU as food is in the digested/hydrolyzed amino acids the protein is made from, and is broken down to when you eat it.
So long as the protein powder you are adding is a complete protein (and not some collagen-based crap, which doesn't have a balanced assortment of the essential amino acids that the human body can't synthesize for itself), it doesn't matter if you're adding raw meat, cooked meat, protein powder, or amino acids (except that amino acids will be absorbed 100%, while proteins - which need pancreatic enzymes to digest/hydrolyze them completely - will be partially malabsorbed) - the value is all in the amino acids they are made up from.
LOL, I wasn't sure when I read people saying that cooking protein powder denatures it... thanks to Diana I now understand thatOops, I posted at the same time as Diana. Her scientific explanation is what I would have said if I were smart enough to verbalize it, lolol. :biggrin:












BTW do you know if cooking with live/cultured products lessens the effectiveness? I cooked some buttermilk I had and wonder if it nullifies its effectiveness. TIA.
Don't feel bad, I though the same thing in the beginning and never added powders to anything over 110 degrees. That was super annoying when I was on liquids and adding it to everything!Thanks for the thorough explanation @DianaCox I read that cooking denatures protein and I didn't really understand what that meant. Thank you for taking the time to explain it so well.
BTW do you know if cooking with live/cultured products lessens the effectiveness? I cooked some buttermilk I had and wonder if it nullifies its effectiveness. TIA.